DR. YUHONG DONG AND MERCURA WANG TIME SEPTEMBER 21, 2022
As the text of the White House Statements on the Human Genome Project in June 2000 stated: “Today we are learning the language in which God created life.” What is our Creator’s language for human lives? Why does it matter? Does the spike protein have a chance to impact them? If so, can we protect them?
The coronavirus disease 2019 (COVID-19), which has caused the worldwide pandemic, is a highly transmissible disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus, just like all other coronaviruses, is characterized by club-like spikes that protrude from its surface.
Spike (S) glycoprotein (“spike protein”) is the largest of four main structural proteins of the SARS-CoV-2. And it is the only cell viral membrane protein responsible for mediating viral entry into the host cell, which is essential for the subsequent viral replication in the human body.
There are a number of studies that indicate spike protein can damage human cell mitochondria, suppress human immune system, form amyloid like proteins, and cause abnormal blood clots.
In this article, we would like to discuss the implications of Spike protein on human health from another perspective, which has been recently reported by a few researchers.
Let us first take a look at a few indicative findings of several studies focusing on COVID-19.
Finding 1: COVID-19 Makes the Brain Age by 2 Decades
A recent British study discovered that the SARS-CoV-2 virus’s ancestral strain (i.e. original strain), which was isolated and sequenced from Wuhan, China, can impair a patient’s cognitive ability in a way equivalent to making the brain age by 20 years.
In this study, which was conducted by experts from the University of Cambridge and Imperial College London Medical School, 46 participants (including 16 mechanically ventilated) that received critical care for COVID-19 in a hospital between March and July 2020 took detailed assessment, in order to evaluate the COVID-19 infection’s cognitive effects in humans.
The researchers discovered a significant decline in the patients’ attention, complex problem solving skills, and memory, along with reduced accuracy, and prolonged reaction time. These cognitive deficits are similar to the decline a person would experience between the ages of 50 to 70, which is equivalent to aging by two decades and/or losing 10 IQ points.
Finding 2: COVID-19 Modifies Human Genes’ Expression
In a study published in 2021 in the Journal of Leukocyte Biology, researchers analyzed genome-wide DNA methylation profiles of peripheral blood from nine terminally-ill COVID-19 patients.
They discovered a distinct DNAm signature of severe COVID-19 disease that showed dramatic cell-type composition changes, hypermethylation of IFN-related genes, and hypomethylation of inflammatory genes.
The study results suggest that the SARS‐CoV‐2 virus can dramatically reshape peripheral blood and lung tissue host immune cell landscapes and may modify cellular DNA methylation (DNAm) states. And SARS-CoV-2 can possibly alter other epigenetic mechanisms such as histone modifications and noncoding RNA.
Our DNA is comprised of a sequence of many genes. Methyl groups (i.e. epigenetic factors) are clusters of hydrocarbons, which attach to strands of DNA in a biological process called DNA methylation (DNAm). DNA methylation regulates gene expression, as methyl groups act as signals along the DNA, turning genetic activities on and off. Therefore, DNAm can change the expression level of a DNA segment without changing its sequence.
Interferons (IFN) are so called because they “interfere” with viruses and prevent them from multiplying. They are proteins that inform our immune system that germs or abnormal cells (e.g. cancer cells) are present in our body, and trigger killer immune cells to destroy them.
Blanco-Melo et al. examined the transcriptional response to SARS-CoV-2 in in vitro infected cells, infected ferrets, and post-mortem lung samples from lethal COVID-19 patients and reported that IFN-I and -III responses are attenuated.
In addition to the level of expression of IFN-I, the timing of the IFN-I response is also a critical factor determining outcomes of infection. An early and potent cellular IFN response is vital for antiviral response, whereas a delayed IFN-I response contributes to pathological inflammation and severe outcome.
Accordingly, a timely early switch “on” of IFN-related genes is a critical factor for the human body to overcome the virus invasion and minimize the severe outcome of the diseases.
Finding 3: Spike Protein Enters Cell Nuclei and Impairs DNA Self-Repair
A research paper published in October 2021 in the journal Viruses stated that the SARS-CoV-2 virus’s spike protein can impair the body’s DNA damage-repair mechanism. The researchers were also surprised to find an abundance of spike proteins in the cell nuclei.
It is well-known that only certain types of proteins can possibly be transported into a human cell nucleus, such as histones, DNA and RNA polymerases, and gene regulatory proteins. The nuclear envelope encloses the DNA by double membranes, and there are complex gatekeepers present in the nuclear membranes to prevent the entry of unwanted substances into the cell nucleus, where most DNA repair occurs.
When DNA is replicating themselves, potential errors can be made. However, fortunately, we have innate DNA self-repairing mechanisms, which act as “guardians of our genes.”
To their surprise once again, the study’s researchers discovered that spike protein significantly suppressed the DNA self-repairing mechanisms, including homologous recombination (HR) and non-homologous end joining (NHEJ).
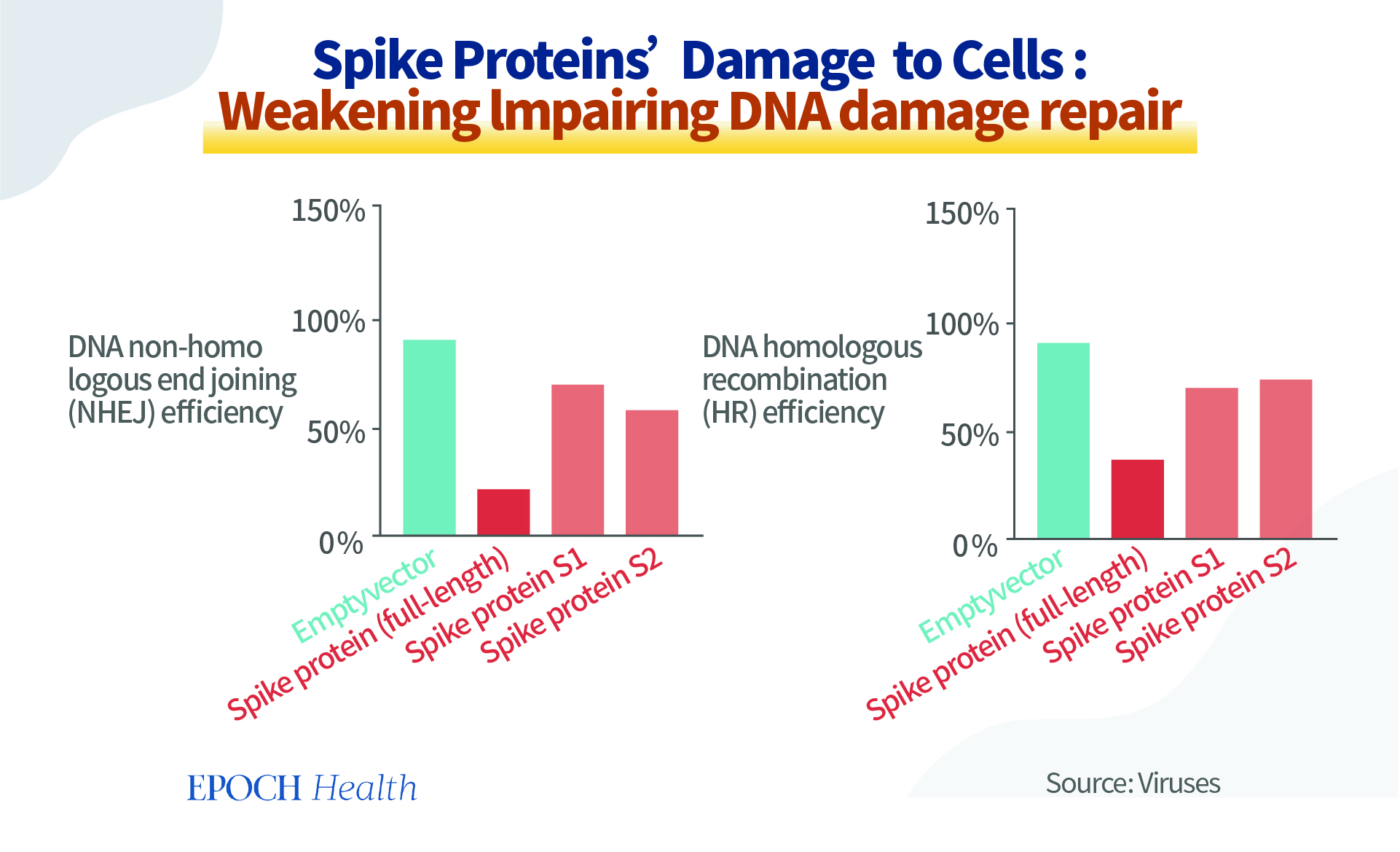
Furthermore, the researchers also found that the spike proteins stayed in the cell nuclei and significantly inhibited DNA damage repair by impeding key DNA repair proteins from gathering at the damage site, and by interfering with double-stranded DNA break (DSB, a principle cytotoxic lesion) repair.
The findings from the aforementioned studies indicate that the spike protein is an unusual protein that can impact the epigenetic function of our human cells.
What Is Epigenetics and Why Does It Matter?
A gene, the basic unit of hereditary material, is a chunk of coding material. It originates from the Greek word γένος (génos), meaning “generation.” The word “epigenetics” is composed of the prefix “epi,” which is derived from the Greek ἐπι (eti), meaning “over” or “around;” and the suffix “genetics.”
Accordingly, epigenetics is the study of heritable changes in gene activity or function that doesn’t involve the alteration of the DNA sequence itself. Similar to gene codes, epigenetics are another type of language used by the Creator to speak with humans.
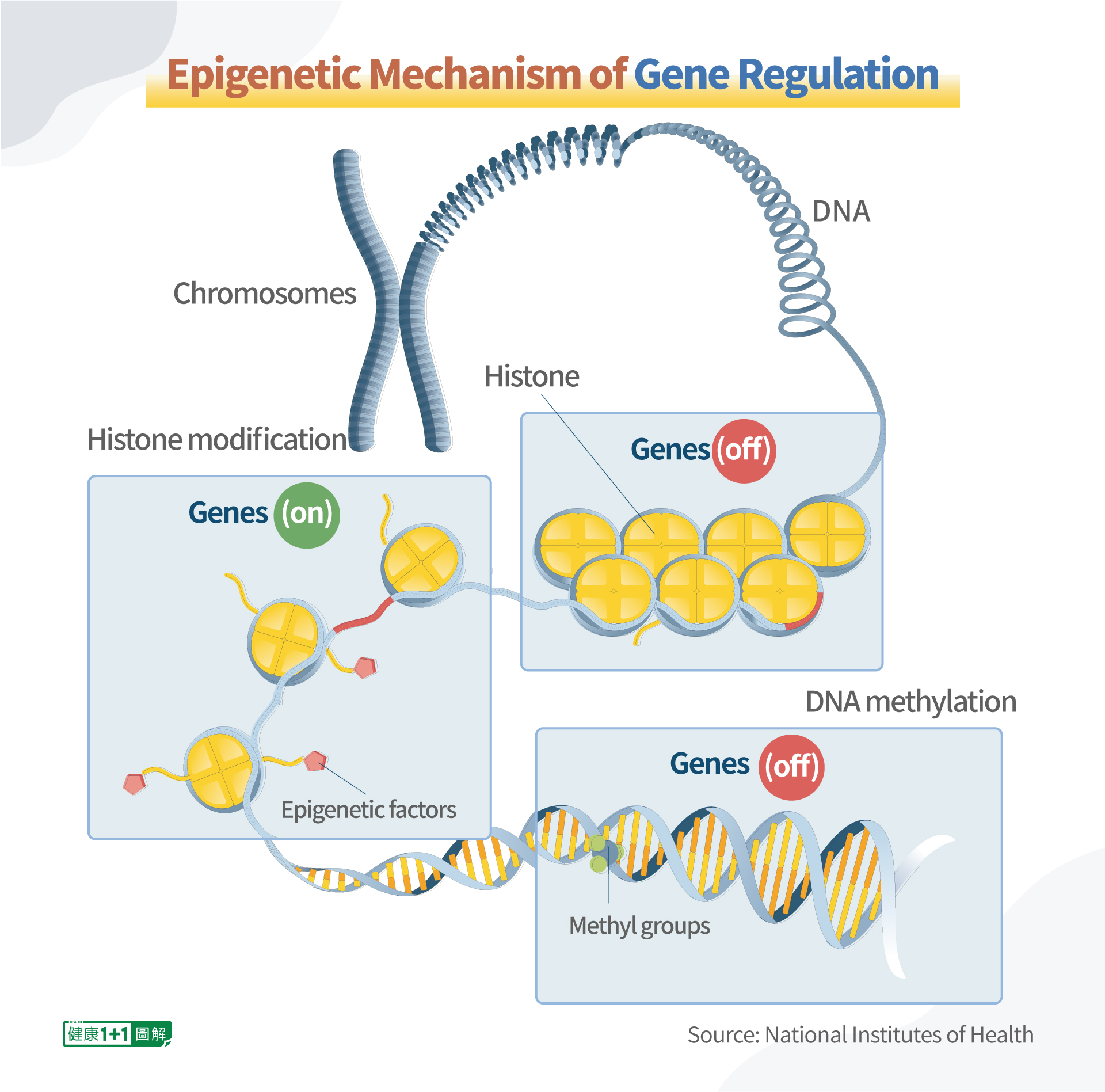
Although virtually all cells in an organism contain the same genetic codes (DNA sequences), they do not express their genes simultaneously or in the same way, so they have totally different functions. That is, the same DNA creates different types of cells such as liver cells, kidney cells, and nerve cells, through the direction of epigenetic factors.
Epigenetic factors (methyl groups) that bind to DNA can directly “turn on” or “turn off” genes. When the genes are “turned on,” they can be expressed and read by the body. Otherwise, they are “turned off” and cannot be read by the body.
Metaphorically, methyl groups are attached to the DNA like “sticky notes.” DNA can be thought of as a script, which can be amended by putting on (or taking off) some sticky notes over its text.
For instance, although all honey bees share the same DNA, researchers have found over 550 genes showing substantial methylation differences between queens and workers. Also, identical twins have the same genome, but they typically don’t have the same personality, characteristics, or even diseases. These differences can be explained by epigenetics.
Thus, DNAm is vital to some cellular processes of our human body, including embryonic development, X-chromosome inactivation, genomic imprinting, and chromosome stability. Abnormal DNA methylation may lead to several adverse outcomes, including malignant tumor, neurological and immunological diseases, atherosclerosis, and osteoporosis.
Changes in epigenetic factors may ultimately determine whether or not a person has a particular disease. So why does the COVID-19 infection cause abnormal aging in the first study mentioned at the very beginning? To answer this question, we have to explore the various impacts of what spike protein, regardless of the virus or the vaccine, could do to human health.
Spike Protein May Interfere Gene Guardians in Various Conditions
- Aging
The aging process is regulated by epigenetic factors. Due to scientific advancements, biomarkers of aging based on DNA methylation data can be used to accurately estimate the age of tissues.
A genome-wide DNA methylation study published in April 2022 in the journal Nature Communications collected whole blood samples from 232 healthy individuals, 194 non-severe COVID-19 patients and 213 severe COVID-19 patients. Researchers discovered that the epigenetic age of COVID-19 patients was significantly accelerated. This result may shed some light on the fact that the COVID-19 infection can make our brain age by two decades.
- Neurodegenerative conditions
In addition, epigenetic modifications have been found to be important players in the pathogenesis of Alzheimer’s disease (AD), which is the most common type of dementia. Extensive research has suggested DNA methylation plays an important role in the course and development of AD.
Since a COVID-19 infection can leave patients with neurological and psychiatric sequelae for a period of time after recovery, a team researchers from the University of Oxford and University of Cambridge performed an analysis of 2-year retrospective cohort studies by examining the medical records of 89 million patients, including both COVID-19 patients and patients with other respiratory diseases at a ratio of 1:1.
Related Coverage
The Pathophysiology of Long COVID
Matching was done on the basis of demographic factors, risk factors for COVID-19 and severe COVID-19 illness, and vaccination status. Analyses were stratified by age group and date of diagnosis. It is a pity that the detailed vaccination data of the study subjects are not well disclosed; whereas the prevalence of vaccination was low in both cohorts, which was probably under-reported in this study.
Nevertheless, it was discovered that throughout the 2-year follow-up period, COVID patients were persistently at an increased risk of psychiatric disorder, cognitive deficit, dementia, and epilepsy or seizures.
- Hematologic malignancies
Recent intensive genomic sequencing of hematopoietic malignancies has identified the central role that aberrant epigenetic regulation plays in the pathogenesis of these neoplasms.
American pathologist Dr. Ryan Cole, founder of Cole Diagnostics, has discovered an abnormal increase in certain cancer cases after the COVID-19 vaccines were introduced, including childhood diseases in adults and rare cancers. At the same time, he has also noted an increase in all-cause deaths among vaccinated individuals compared to unvaccinated individuals.
In a similar situation, after COVID-19 vaccination started to be implemented in mainland China, as of early June 2022, at least 845 people had been reported to have hematological malignancies, with an age range from 1 to 80 years.
Furthermore, coexisting COVID-19 infection and hematological disorders have also been shown by several reports. The authors of a study published in the journal Archives of Academic Emergency Medicine were greatly concerned by the possible link between the COVID-19 infection (or the medicines used in the treatment) and the risk of developing acute leukemia.
- Myocardial injury
According to multiple studies, the association between both long noncoding RNAs and microRNAs and the development of cardiovascular diseases has been confirmed. RNA (ribonucleic acid) plays a central role in turning genetic information into proteins in our body.
As per a British study with participants over 13 years of age, the risk of myocarditis is greater after SARS-CoV-2 infection than after COVID-19 vaccination. However, the risk of myocarditis after vaccination is higher in young men. Some of the vaccines used in the UK are mRNA-based, including Pfizer and Moderna vaccines.
- Autoimmune conditions
The link between epigenetics and autoimmunity has already been well-documented in scientific literature. Epigenetic changes, such as DNA methylation and noncoding RNAs, have been discovered to play a role in the pathogenesis of autoimmune diseases, mainly by regulating gene expression.
The medical community is becoming increasingly aware of vaccine-induced autoimmune diseases involving liver, heart, and nervous system, among others.
In a case published in April 2022 in the Journal of Hepatology, a 52-year-old German man developed acute hepatitis twice after receiving two doses of Pfizer mRNA vaccine. He was tested positive for autoimmune markers, and his doctor later noticed a strong correlation between the onset of hepatitis and his vaccinations, which made the latter suspect that the liver injury was caused by the vaccine.
In another case from Spain, a 41-year-old woman, who received the Moderna mRNA vaccine, was diagnosed with vaccine-induced autoimmune liver injury, along with severe cholestasis.
Molecule of Silence: Gene Guardian Protectors
Based on the vaccine induced multiple systemic adverse events and basic research on spike protein, all of this has indicated that the spike protein is likely to play a role in damaging our gene “guardians”–our DNA self-repair mechanisms.
Currently, a majority of the world population has received at least one dose of a COVID-19 vaccine and the 3rd and 4th booster program is being proposed again, regardless of the fact that the recent mutant virus strains have become less lethal.
Under these circumstances, in order to protect our health better, what can we do to better protect our gene guardians, so as to protect our genes?
As the text of the White House Statements on the Human Genome Project in June 2000 stated, “Today we are learning the language in which God created life.” Both genes and epigenetics are our Creator’s language for human lives. To protect them, we may have to rediscover our traditional ways of living, as the tradition is our Creator’s guidance on how to live healthily.
Mindful practices have long been suggested to promote well-being by producing a state of body relaxation and inner silence, i.e., a state of quiet mind and emotions characterized by the absence of recurring thoughts, images, and emotional fluctuations.
Mind-body therapies (MBTs), such as mindfulness, sitting in meditation, yoga, and tai-chi, have been proven to be able to improve our quality of life by reducing stress. MBTs have also been discovered to epigenetically affect our genes.
For instance, studies have found that the methylation of the tumor necrosis factor gene can significantly decrease among women who perform yoga. People who sit in meditation can experience a significant alteration in various modifications of histone deacetylase enzymes and their gene expression patterns. Long-term meditators can even enjoy the benefit of having slower biomarkers of aging, and methylation in genes associated with immune cell metabolism and inflammation. Therefore, MBTs can potentially serve as therapeutic treatments and preventative measures used to affect the epigenetics of an individual, as well as an addition to Western medicine.
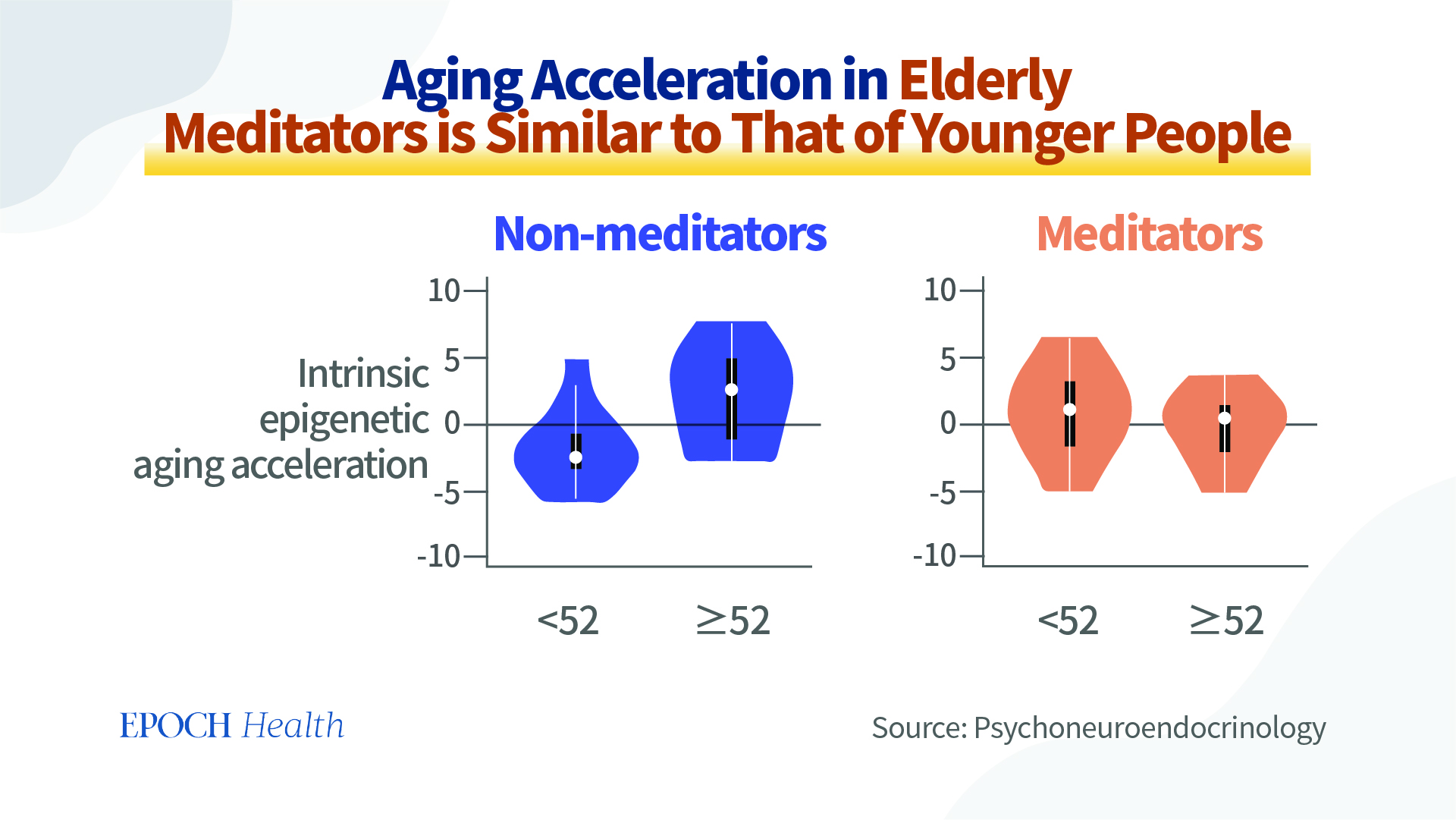
In 2021, the World Health Organization (WHO) also recommended meditation as a rehabilitation method for COVID-19 patients. Also, there seems to be a degree of international consensus that meditation is helpful for people recovering from the side effects of COVID-19 vaccines and COVID-19 sequelae (pdf).
Meditation has also been found to have the following benefits:
- activating specific brain regions;
- increasing heart rate variability;
- suppressing inflammation;
- increasing telomerase expression, which affects the body’s aging mechanism.
According to a large-scale genome study published in the Proceedings of the National Academy of Sciences (PNAS), meditation activated the participants’ immune system, with a total of 220 immune genes being upregulated, including 68 genes related to interferon and belonging to the innate immune mechanism. In this study, there is a highly likely role of meditation on these gene guardians.
Therefore, in order to better protect our gene guardians, we should give mind-body therapies a try.
References
Coronaviruses: An Updated Overview of Their Replication and Pathogenesis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8013321/?report=reader
DNA Methylation and Its Basic Function | Neuropsychopharmacology
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7227586/?report=reader
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7104995/?report=reader
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7402635/?report=reader
SARS–CoV–2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro
DNA Methylation and Its Basic Function | Neuropsychopharmacology
The Honey Bee Epigenomes: Differential Methylation of Brain DNA in Queens and Workers – PMC
Epigenetics of discordant monozygotic twins: implications for disease | Genome Medicine | Full Text
DNA hypermethylation in disease: mechanisms and clinical relevance – PMC
DNA methylation-based biomarkers and the epigenetic clock theory of ageing | Nature Reviews Genetics
Accelerated biological aging in COVID-19 patients | Nature Communications
Epigenetics of Alzheimer’s Disease – PMC
https://genesdev.cshlp.org/content/30/18/2021.long
Dr. Ryan Cole: Alarming Cancer Trend Suggests COVID-19 Vaccines Alter Natural Immune Response
The novel regulatory role of lncRNA‐miRNA‐mRNA axis in cardiovascular diseases – PMC
The emerging role of epigenetics in human autoimmune disorders
SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis – Journal of Hepatology
Coronavirus (COVID-19) Vaccinations – Our World in Data
The code, the text and the language of God – PMC
Molecules of Silence: Effects of Meditation on Gene Expression and Epigenetics
The potential positive epigenetic effects of various mind-body therapies (MBTs): a narrative review
Epigenetic clock analysis in long-term meditators – PMC
Support for rehabilitation self-management after COVID-19-related illness
